The growing acknowledgment and acceptance of data generated by electronic clinical outcomes assessment (eCOA) platforms by regulatory agencies contributes to a valuable advantage for sponsors—enabling faster regulatory approvals and expediting time-to-market in an inherently competitive environment. In clinical studies, eCOA offers a range of benefits, including improved data accuracy, enhanced participant engagement, greater site personnel productivity, and streamlined study processes. This is why eCOA adoption continues to increase despite some growing pains, which are already being addressed by advanced technologies and enhanced user training.
Increasingly complex studies require researchers and clinicians to monitor patient progress more closely, make timely decisions, and respond promptly to symptom reports that may require adverse event evaluations. The weight of these ever-increasing requirements is daunting. eCOA excels in capturing subjective experiences and symptoms, providing valuable insights into the impact of treatments from the patient’s perspective. eCOA also allows for real-time data collection and remote monitoring, providing immediate access to patient-reported outcome measure data.
To gain insights into the current state of eCOA as well as industry expectations, YPrime conducted a survey of clinical professionals to discover their perspectives on eCOA technology platforms. Here are some key findings that were revealed.
- Timelines and data quality: The top concerns that repeatedly surfaced in the survey results include timelines/delays, data quality, data change capabilities, protocol amendments, tech flexibility, and technology ease-of-use.
- Measures of success: Survey respondents site visit compliance, diary/scale compliance, and patient enrollment status as the top indicators of endpoint collection success.
- Integration with other technologies: Use of wearables and sensors in clinical research is increasing, and clinical professionals expect them, as well as other eClinical systems, to integrate with their eCOA platform for greater insights.
- eCOA provider relationship: The clinical operations team primarily drives the selection of eCOA providers, with data quality, user interface/ease-of-use, and faster study start-up being important considerations. The burden of switching eCOA providers includes establishing new third-party integrations, procurement qualification of the new provider, and the loss of customizations with the incumbent eCOA provider.
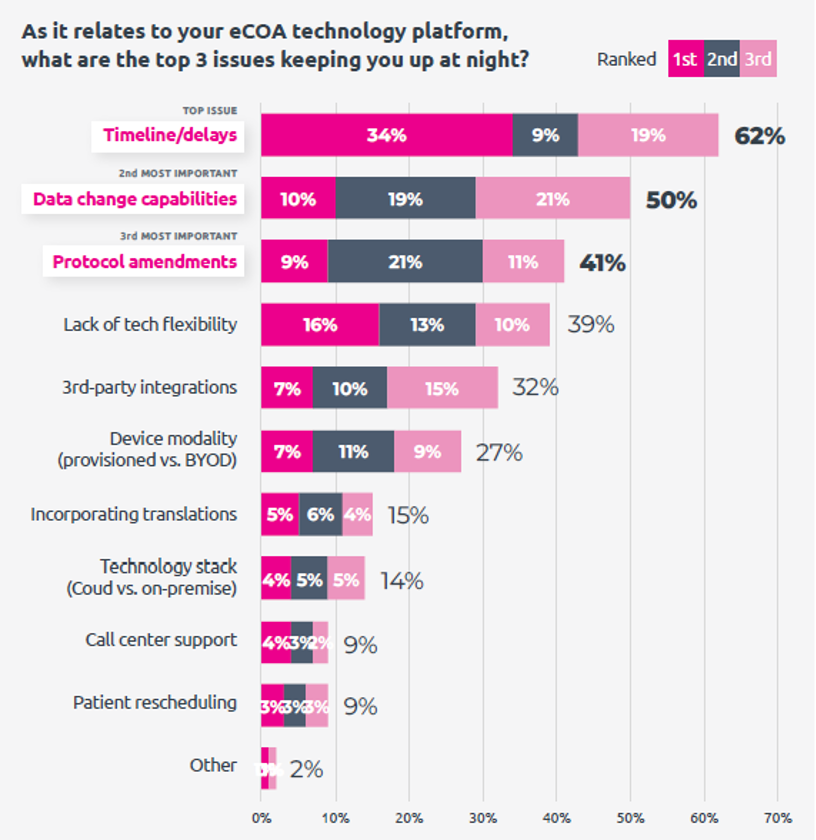
One of YPrime’s keys to “Solving for Certainty” is to continually conduct research with all our stakeholders, so that we can provide the solutions that will truly improve their experiences and help accelerate research. I invite you to read the full eCOA Trends for Today and Tomorrow report, as well as the other resources you can find on our website.
