Across the clinical trial industry, the perception of electronic informed consent, or eConsent, often falls into two categories: an oversimplification equating it to electronic signatures, or an overcomplication seen as too burdensome for understaffed research teams.
In reality, the ideal eConsent solution is achievable and comprehensive. It considers factors like technology, compliance, patient accessibility, and usability. While this necessitates a complex platform, experienced clinical trial technology vendors leverage user-centric design from consumer-based technologies to streamline the patient experience and enhance data collection.
Traditional, paper-based consent struggles to keep pace with the growing complexity of trials and the integration of digital tools. eConsent replaces paper-based processes with interactive, educational models. By presenting information in digestible chunks with visual elements, this technology improves comprehension and engagement—which translates to better informed patients and smoother study activities. As the technology evolves, it will offer even greater value for complex clinical trials.
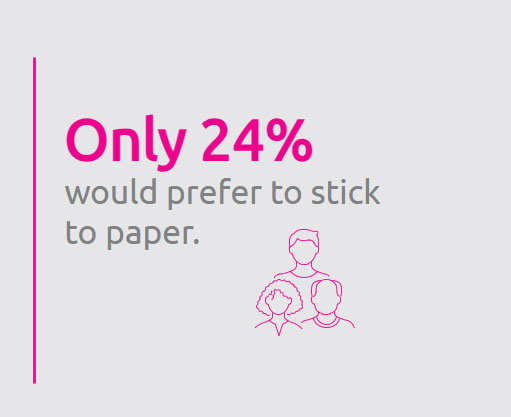
A 2023 article published in the Journal of Medical Internet Research indicates that “compared with patients using paper-based consenting, patients using eConsent had a better understanding of the clinical trial information, showed greater engagement with content, and rated the consenting process as more acceptable and usable.”1
As industry adapted to the COVID-19 pandemic, we saw an acceleration in the adoption of eConsent, but there are still some that question what the difference is between eConsent and a pdf review technology (such as DocuSign). While DocuSign offers a basic solution, eConsent goes beyond replicating paper documents. It allows for interactive and user-friendly formats that enhance patient understanding of the study and their role within it.
Ideally, eConsent serves as another tool for clinical trial teams, enabling them to tailor the consent process to each participant’s comfort level and the specific trial requirements. This can range from simple electronic signatures to guided documents designed for optimal user experience.
from YPrime report, January 2024, Clinical Trial Technology — What Patients Want, and What You Should Avoid.
Improved Consent for Better Outcomes
eConsent is gaining momentum globally. As the need for these technologies grows, establishing clear goals becomes crucial. These goals mirror the traditional consent process, with enhancements made possible by advanced technology. They include:
When evaluating an eConsent solution, the prioritization of patient comprehension cannot be undermined. Technology providers must create an engaging experience that surpasses the limitations of paper forms. This can involve incorporating videos, interactive text, knowledge assessments, and the ability to flag sections for provider review.
Forget dry, text-heavy documents—the future of eConsent lies in user-friendly interfaces that are intuitive and appealing, mirroring the best practices of consumer technology.
Flexible eConsent: Meeting Patients Where They Are
eConsent goes beyond just electronic consent. It provides flexibility. It empowers sites and users to obtain consent on-site, remotely, or through a combination of both. The core principle is to meet patients where they are most comfortable.
This “user-first” approach is essential for building a patient-centric experience. It requires a meticulous examination of every step in the consent process, from onboarding to signature, to ensure an exceptional user experience.
Optimizing eClinical Workflows
Recruiting potential study participants is a significant investment for trial sponsors and teams. A smooth and engaging onboarding experience is crucial for maintaining momentum and improving retention. This means identifying and eliminating friction points and prioritizing ease of use at every stage.
Balancing Patient and Site Needs
For site users, managing multiple clinical trial applications across various studies can be overwhelming. Considering the site burden when developing eConsent technology is crucial. Key questions include:
While focusing on the patient experience is important, developers should not neglect the site user experience or the technology’s role within the broader digital ecosystem.
Streamlining the Clinical Trial Journey
eConsent aims to minimize burden for both patients and sites, improving the consent experience and ultimately reducing attrition. By simplifying integration and communicating the value of eConsent, sponsors can encourage wider adoption among researchers and providers, leading to more standardized application across trials.
This, in turn, fosters process improvements like better version control, data integrity, reduced administrative burden, and faster recruitment insights. Designing eConsent technologies that seamlessly integrate with a trial team’s workflow, while remaining intuitive for participants, is key to a successful clinical trial journey.
At YPrime’s Innovation Network Gathering, Mike Wenger, CEO and Founder of VersaTrial, compared innovators to mountain climbers and pharma company champions to Sherpas. In this analogy, Mike offers guidance on conquering clinical trials and delivering life-saving treatments faster. Read the blog post and watch a video of Mike’s talk: Disrupting Clinical Research: A Guide to Streamlining Trials.
Source
- (2023) Comparative Effectiveness of eConsent: Systematic Review, Journal of Medical Internet Research ↩︎
Check out our other patient engagement resources
about trial design, data capture, operational efficiencies, and, ultimately, solving for certainty in clinical research.