Oncology clinical trials are uniquely complex. Clinical research teams have the important job of finding ways to make trials flow as quickly and smoothly as possible, delivering a compassionate experience to patients, and a manageable experience to site staff—all while ensuring the data collected is of the highest quality, and ready for regulatory submission. With 44,704 ongoing clinical trials involving oncology, the importance of timelines, and quality data cannot be underestimated.1
In a recent survey of industry professionals, several concerns surfaced regarding the challenges faced in conducting oncology clinical trials. YPrime’s Oncology Infographic shows the top concerns—and recommendations—on how to solve for certainty when choosing an eCOA provider for oncology clinical trials.
Flexible clinical technologies, such as eCOA, address unique challenges in clinical research such as diverse treatment modalities, and complex endpoints. As we navigate oncology research, technologies must be streamlined and user-centric to improve clinical trial outcomes.
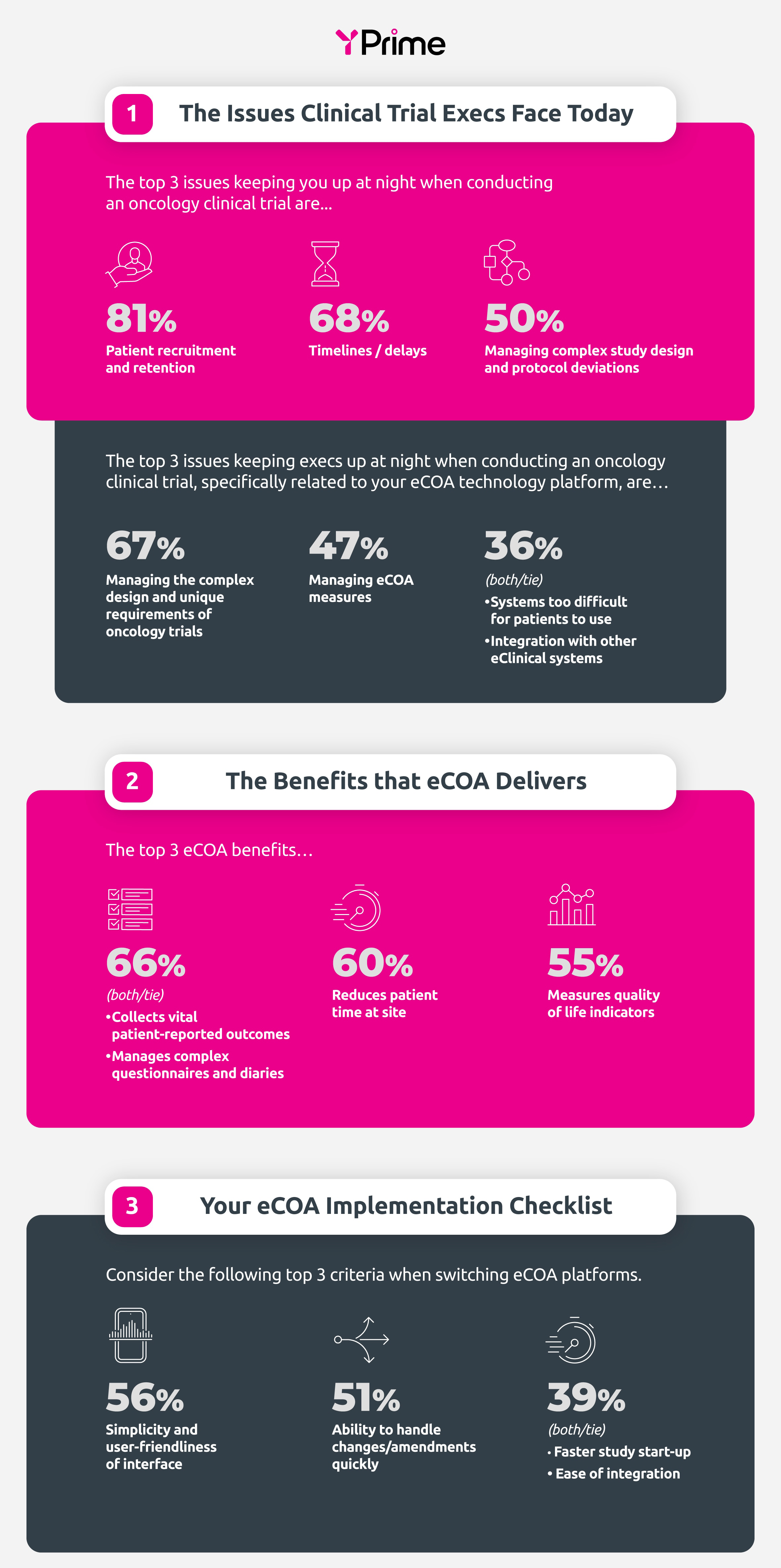
Source: Data from YPrime Survey, 2023.
All data presented in this infographic are from the report, Decreasing the Burden of Oncology Clinical Trials with eCOA, issued by YPrime in March 2024. The full report may be found at www.yprime.com/resources.