With millions worldwide affected by neurological conditions, the importance of accelerating therapies has never been more pressing. According to the U.S. National Library of Medicine, there are more than 600 neurologic diseases, including a wide range of disorders, such as Alzheimer’s disease, epilepsy, learning disabilities, neuromuscular disorders, autism, ADD, brain tumors, and cerebral palsy, just to name a few.1
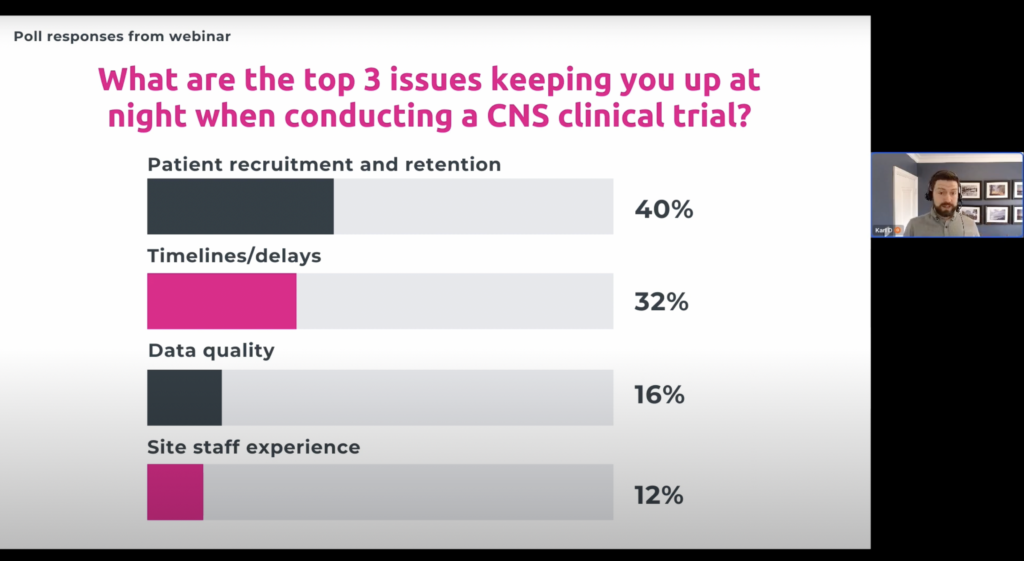
In a recent survey of executives conducting CNS clinical trials, YPrime inquired about the challenges they face with eCOA, uncovering a number of concerns the industry faces.
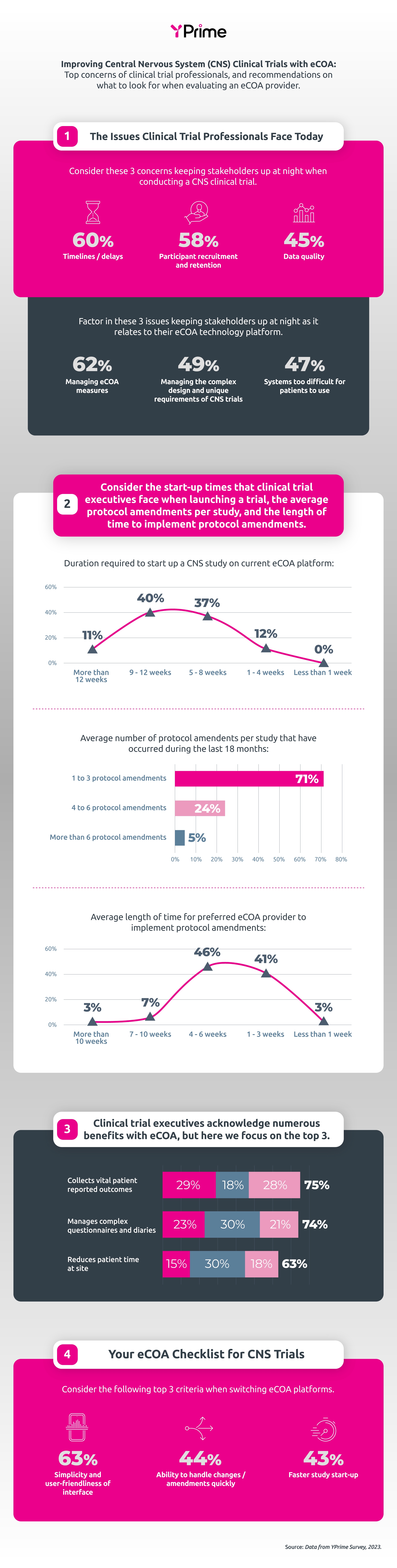
The top concerns involve managing eCOA measures, managing complex design, in addition to some systems being too difficult for patients to use. Prioritizing a simple and easy-to-use interface, along with configurability to handle protocol amendments quickly, can alleviate some of these burdens. With more than half of respondents stating that it takes 4 weeks or greater for their eCOA provider to implement protocol amendments, the importance of configurability and faster system changes cannot be underestimated.
YPrime’s CNS Infographic shows the top eCOA concerns, along with recommendations on what to look for when evaluating an eCOA provider for CNS clinical trials.
eCOA EMPOWERS CNS CLINICAL TRIALS WITH SPEED, FLEXIBILITY,
AND CERTAINTY.
Flexible and configurable technologies are essential in addressing the intricate challenges presented by CNS disorders, ensuring data quality for complex endpoints. As we navigate CNS research, technologies must be streamlined and user-centric to enhance clinical trial outcomes.
At YPrime, our eCOA platform empowers trial execs to solve for certainty in clinical research with flexibility and speed. From seamless data collection to efficient endpoint collection, our innovative solutions streamline processes, allowing you to focus on advancing research and improving patient outcomes.
Check out our other CNS resources
about trial design, data capture, operational efficiencies, and, ultimately, solving for certainty in clinical research.