Dermatology researchers are innovating both treatments and research processes. Despite FDA approval of over 110 drugs for about 30 conditions1, many skin problems still lack effective solutions, underscoring the need for ongoing research and improved clinical trial methodologies.
A recent YPrime survey reveals key challenges in dermatology research. Participant recruitment remains a top issue, while integration with other clinical systems is the primary tech concern for electronic clinical outcome assessments (eCOA). 52% of professionals surveyed express concern about timelines and delays. 56% of respondents face startup times of nine weeks or longer, and 54% report their eCOA provider takes over four weeks to implement protocol amendments.
eCOA plays a vital role in ensuring data quality, patient involvement, and addressing unique challenges like accurate visual data capture and maintaining patient compliance. As dermatology trials evolve, so does supporting technology. The infographic below illustrates key concerns and offers recommendations for selecting eCOA providers, helping sponsors navigate this complex landscape more effectively.
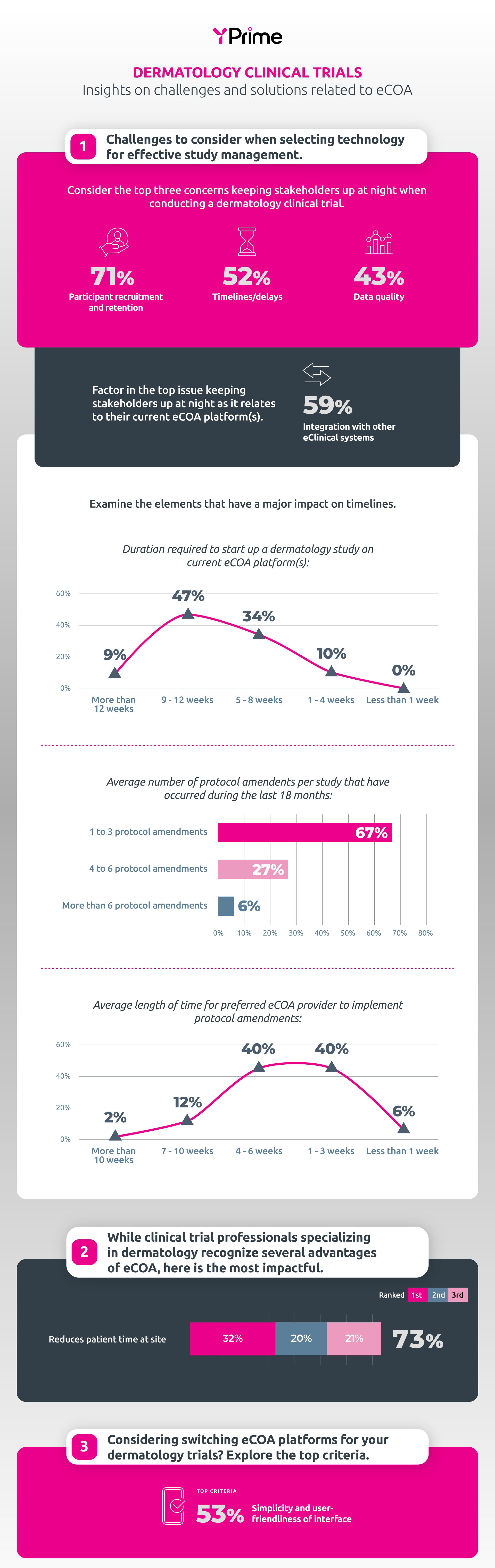
Source: Data from YPrime Survey, 2023.
Harnessing eCOA: The Future of Dermatology Trials
Thorough evaluation through clinical trials ensures the efficacy and safety of new treatments and drives progress toward comprehensive solutions for skin health and chronic condition management. Digital technologies like eCOA can help by providing real-time, accurate data collection and patient feedback. This technology enhances the reliability of trial results and facilitates better management of patient-reported outcomes. By integrating eCOA into dermatology trials, researchers can better understand treatment effects and patient experiences, leading to more effective and personalized dermatological care.
Take a look at our CNS Infographic to get more insights on the industry’s top eCOA concerns and recommendations on what to look for when evaluating an eCOA provider for CNS clinical trials.
Check out our other eCOA resources
about trial design, data capture, operational efficiencies, and, ultimately, solving for certainty in clinical research.