Clinical Trial Technology — What Patients Want, and What You Should Avoid.
The rise of patient centricity in clinical research is an evolution driven by ethical considerations, regulations, technological advancements, and the critical contributions patients bring to the research process. As technology evolves, industry leaders need to know—how do patients feel about the technologies they are being asked to use? Do clinical trial participants prefer paper or digital tools for informed consent and patient diary entries? What are the biggest pain points to avoid to improve patient retention and trial completion?
Patient Perspectives on Clinical Trial Technology
Informed Consent and Patient Diaries:
The Study Participant’s Point of View
Download the white paper to discover:
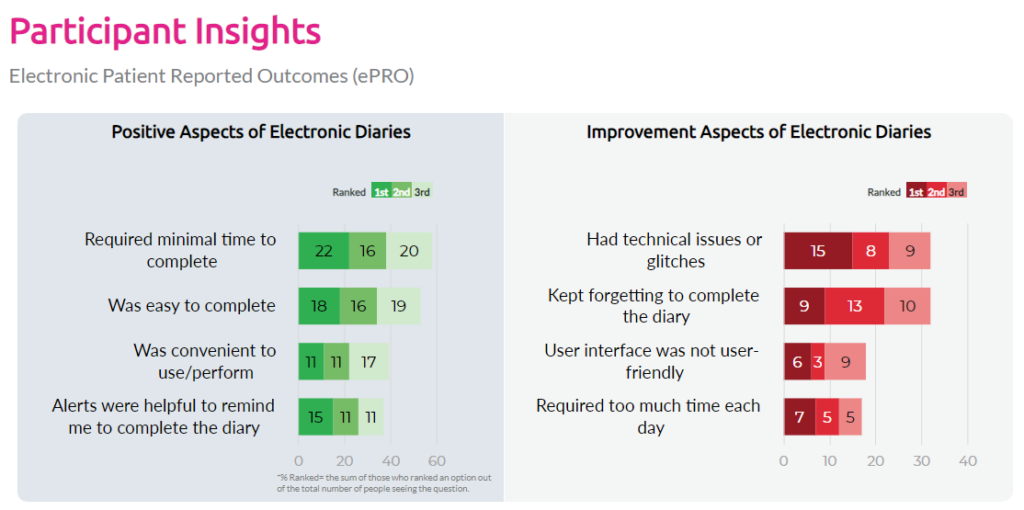
Are eDiaries the path forward for patients?
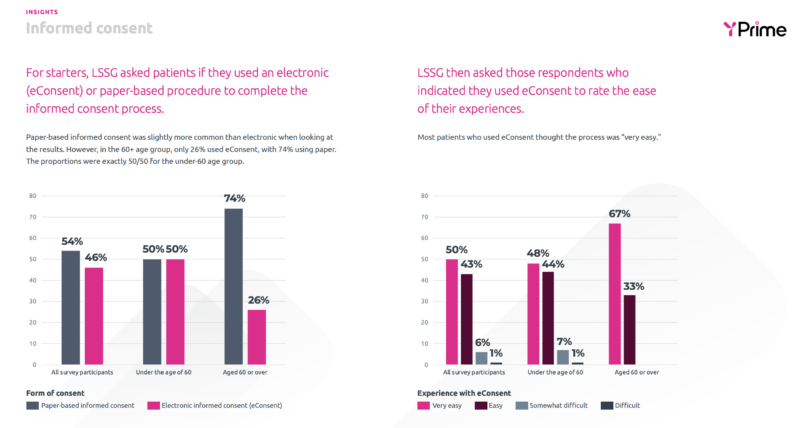
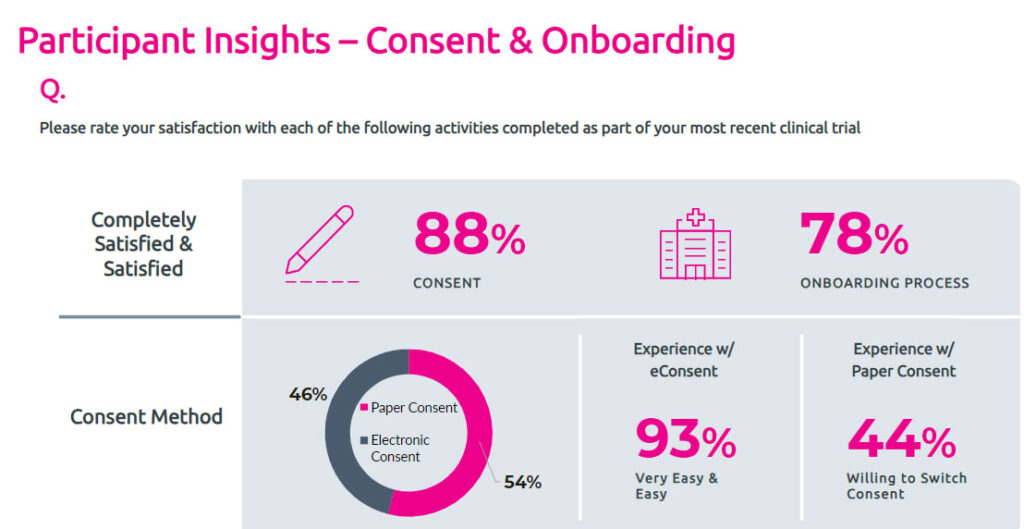
Do clinical trial participants find eConsent easy or difficult to use?
What tech improvements do patients recommend?
A Glimpse at the Report


Author
Author

Drew Bustos
Chief Marketing Officer.
YPrime

©2024 Y-Prime, LLC.All Rights Reserved.