In oncology clinical trials, selecting the right electronic clinical outcome assessment (eCOA) platform is critical to ensuring trial success. With complex protocols, diverse patient populations, and stringent regulatory requirements, having the right solution is of the utmost importance. This blog will help you as you search for an eCOA provider for your oncology trials, focusing on the importance of seamless technology and patient-centricity.
1. Prioritize a Patient-Centered eCOA Design
In oncology trials, the patient experience isn’t just important—it’s essential. A patient-centered eCOA platform must go beyond functionality; it should be intuitive, accessible, and designed with the patient’s needs in mind. When evaluating a new eCOA vendor, look for platforms that feature a streamlined, user-friendly interface with clear instructions and seamless navigation across all devices, so patients can easily participate no matter where they are.
The more intuitive the platform, the more likely patients will engage and comply, directly impacting the reliability and accuracy of your data. Advanced features like multi-language support, automated reminders, and real-time notifications help keep patients motivated, especially in global clinical trials. By focusing on making the patient experience as simple and supportive as possible, you’re not only enhancing compliance but also ensuring the integrity of your data, ultimately leading to more successful and actionable trial outcomes.
2. Ensure Flexibility for Evolving Oncology Protocols
Oncology trials are dynamic by nature, with protocols often evolving throughout the study. Whether due to changing endpoints, regulatory updates, or patient needs, the ability to adapt is crucial to maintain trial momentum. That’s why choosing an eCOA solution with built-in flexibility is essential.
A robust eCOA platform should allow seamless adjustments to workflows, ensuring protocol changes can be implemented swiftly without disrupting trial operations. The best eCOA providers offer solutions that are agile and can accommodate complex, real-time protocol modifications while maintaining data integrity. This adaptability minimizes delays and ensures that your trial remains on track, meeting both regulatory standards and operational goals.
By selecting a flexible eCOA vendor, you can stay ahead of protocol shifts and ensure that your oncology trial runs smoothly—no matter what challenges arise.
3. Ensure High Data Quality and Regulatory Compliance
In all clinical trials, data quality is critical. To safeguard the success of your trial, choose an eCOA solution that guarantees accurate, real-time data collection from the start. The best eCOA platforms offer advanced features like real-time monitoring, automated compliance tracking, and customizable, easy-to-interpret reports.
These tools help you stay audit-ready at all times, meeting the strict regulatory standards required for oncology research. With real-time analytics, you can quickly identify potential risks and adjust course as needed, minimizing delays and maintaining the integrity of your trial data.
By selecting an eCOA provider that emphasizes data quality, you ensure smoother trial operations and faster, more efficient outcomes—all while keeping compliance and data integrity intact.
4. Partner With a Trusted eCOA Provider
When selecting an eCOA provider, experience is crucial. Choose vendors with a proven track record in oncology trials and a deep understanding of the unique challenges these studies present. A trusted eCOA vendor will offer comprehensive support at every stage of the trial, from initial platform setup to ongoing management and troubleshooting.
The best eCOA providers ensure smooth integration with other clinical systems, streamlining the trial process and minimizing operational disruptions. By partnering with a vendor that brings both expertise and seamless support, you can navigate the complexities of oncology trials with confidence and efficiency.
5. Embrace Innovation: Leverage Connected Devices in Oncology Trials
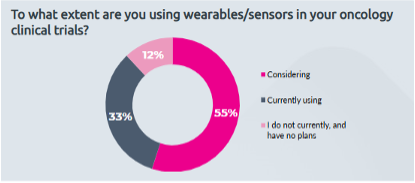
Innovation in eCOA solutions is rapidly evolving. An exciting development is the integration of connected devices, like wearables and remote monitoring tools. Despite their advantages, only 33% of oncology clinical trial sponsors are currently using wearables, with another 55% considering their use, according to YPrime research. By embracing connected devices, you can collect real-time health data that enhances patient engagement and improves data accuracy.
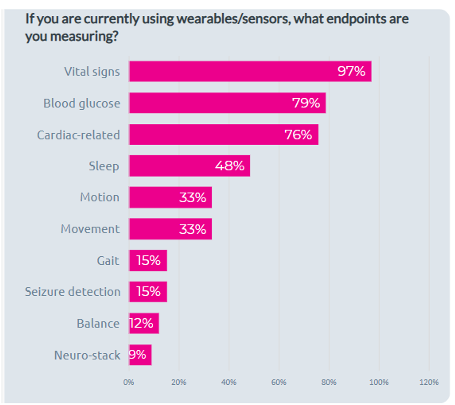
YPrime’s research shows that the top three oncology endpoints measured with connected devices are vital signs, blood glucose, and cardiac health, immediately followed by sleep and motion. In the years to come, connected devices will capture more continuous, passive data from patients. This improves patient adherence and provides valuable insights into health trends. Incorporating devices into your eCOA solution enhances your data collection and ensures real-time feedback, improving both trial efficiency and patient outcomes.
Why Choosing the Right eCOA Provider is Crucial for Oncology Trials
The success of an oncology trial depends on accurate data, patient engagement, and smooth operations. By choosing the right eCOA solution, you ensure all these factors are in place. Whether through patient-centered design, flexible protocols, high-quality data, or the use of connected devices, a leading eCOA provider will help your oncology trial succeed.
With innovations like wearables and connected devices, oncology trials are becoming more data-driven and patient-friendly. Select an eCOA provider that embraces these advancements, and your trial will be set up for success.
YPrime conducted the eCOA Trends in Oncology Clinical Trials survey in December 2023. The aim was to discover perspectives on eCOA technology platforms from clinical trial professionals at biotechnology and pharmaceutical companies specializing in oncology research. The full report can be accessed here: https://www.yprime.com/wp-content/uploads/2024/04/eCOA-for-Oncology-Survey-Report-YPrime.pdf.
Check Out Our Other eCOA Resources
about trial design, data capture, operational efficiencies, and, ultimately, solving for certainty in clinical research.



