Clinical Trials
Run on Certainty
Speed, flexibility, and oversight are built into every part of your eCOA, IRT, eConsent, and participant-engagement workflows. In oncology, rare disease, CNS, cardiometabolic, vaccines, ophthalmology, and across complex global studies, YPrime gives sponsors and CROs the confidence to execute with certainty.
With configurable architecture, integrated data flows, and consistent global delivery, YPrime helps clinical trial teams move faster, from study startup through closeout—while eClinical technology that enables data quality at every step.
CLINICAL TRIAL TECHNOLOGY BUILT TO
Increase
speed
Accelerate clinical trial startup, mid-study changes, and global execution across eCOA, IRT, and eConsent.
Empower
flexibility
Adapt protocols, strategies, and study designs without disruption, supporting today’s adaptive clinical trials.
Maintain
oversight
Ensure inspection-ready data, traceable workflows, and accountable review across eCOA, IRT, and eConsent.
eClinical Technology for Faster Study Startup, High-Quality Data, and Confident Execution.
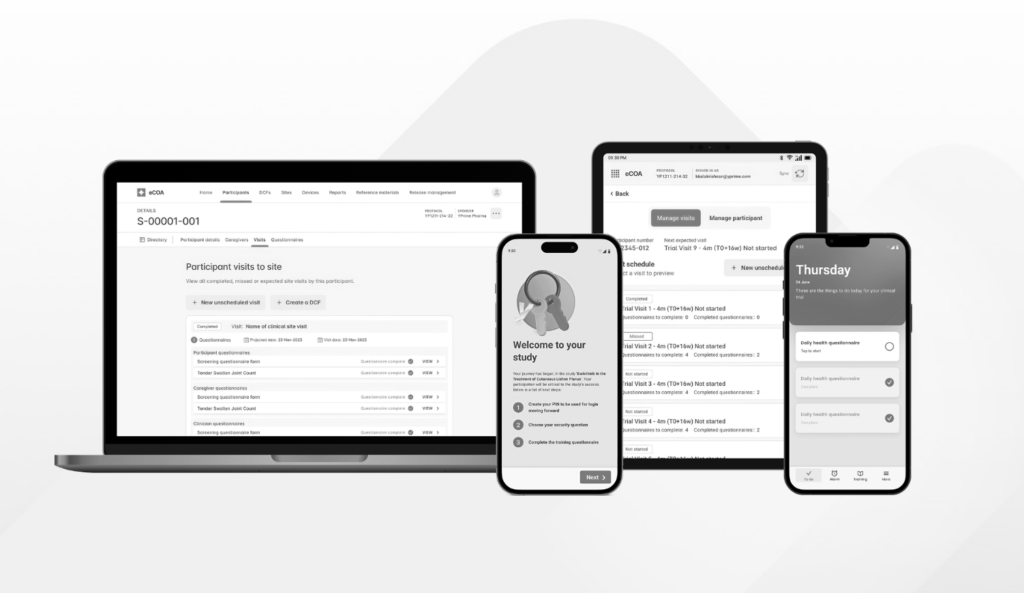
YPrime’s eCOA, IRT, and eConsent solutions are built for global scale and complex clinical trial protocols. Our eClinical technology helps sponsors and CROs move faster while maintaining data integrity, oversight, and regulatory confidence.
IRT startup times.
Protocol amendments.
eCOA with pre-validated code.
YPrime supports top pharmaceutical companies, emerging biotechs, and CROs with a modern clinical trial platform that simplifies execution, reduces site burden, and improves participant experience—without sacrificing data quality or compliance.
Looking for Ideas and Inspiration
to Improve Your Clinical Trial Strategy?
Access expert insights, eCOA and IRT resources, case studies, and thought leadership designed to help sponsors and CROs run more efficient, compliant, and patient-centric clinical trials.